Sitemap
A list of all the posts and pages found on the site. For you robots out there is an XML version available for digesting as well.
Pages
Posts
Blog Post number 4
Published:
This is a sample blog post. Lorem ipsum I can’t remember the rest of lorem ipsum and don’t have an internet connection right now. Testing testing testing this blog post. Blog posts are cool.
Blog Post number 3
Published:
This is a sample blog post. Lorem ipsum I can’t remember the rest of lorem ipsum and don’t have an internet connection right now. Testing testing testing this blog post. Blog posts are cool.
Blog Post number 2
Published:
This is a sample blog post. Lorem ipsum I can’t remember the rest of lorem ipsum and don’t have an internet connection right now. Testing testing testing this blog post. Blog posts are cool.
Blog Post number 1
Published:
This is a sample blog post. Lorem ipsum I can’t remember the rest of lorem ipsum and don’t have an internet connection right now. Testing testing testing this blog post. Blog posts are cool.
awards
Danish Government Scholarship (2019 - 2021)
Awarded on:
Awarded for a Masters of Engineering in Mechatronics (profiling in Embedded Systems)
The Danish Government Scholarship, awarded by the University of Southern Denmark, fully covered the tuition fees for my two-year Master’s program in Engineering, specializing in Embedded Systems. In addition to the tuition coverage, the scholarship provided a monthly stipend to support living expenses throughout the duration of my studies. This award facilitated my academic and professional development in the field of embedded systems, distributed systems and IoT, allowing me to focus on research and practical applications in an advanced engineering environment.
experience
Software Developer at NIBE
Employment Period: -
Role: Embedded Software Developer at JPI-NIBE, the in-house software consulting arm of NIBE Group.
Software Developer at Oticon
Employment Period: -
Role: Embedded Software Developer within the eSoftware Department at Oticon.
Software Consultant at Pharma IT
Employment Period: - Present
Role: Software Consultant specialized in digital transformation and AI-driven data management solutions within the Pharma Industry.
portfolio
IDMP Gap Analysis Tool
Duration: - Present
As a Software Consultant at Pharma IT, I designed and developed the IDMP Gap Analysis Tool, a progressive web application aimed at providing a GxP-compliant solution for the pharmaceutical industry. The application facilitates the management and submission of medicinal product data in compliance with the Identification of Medicinal Products (IDMP) standards set by the European Medicines Agency.
IoT Solution for HVAC Systems(Master’s Thesis Project)
Duration: -
For my Master’s thesis, I developed an IoT solution for HVAC systems in collaboration with NIBE to enable existing HVAC modules to connect to the cloud and be controlled remotely. 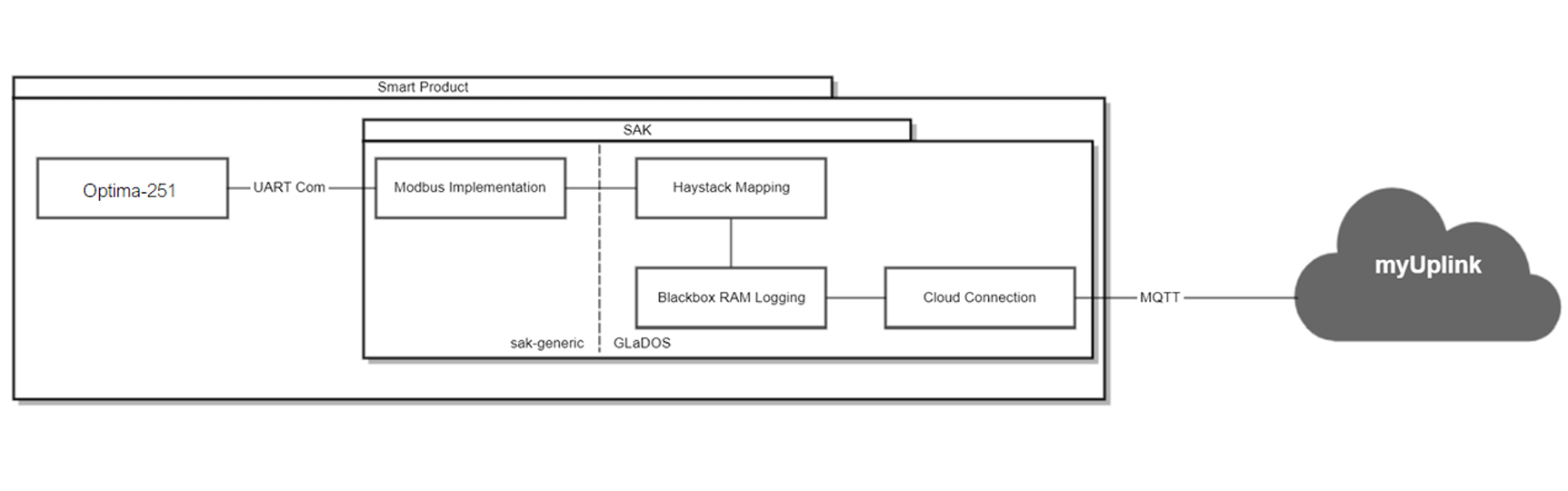
GSM Home-Based Security System(Bachelor’s Thesis Project)
Duration: -
For my Bachelor’s thesis, I designed and implemented a GSM-based home security system using an Arduino Uno and a GSM 900 module to provide a cost-effective home security solution.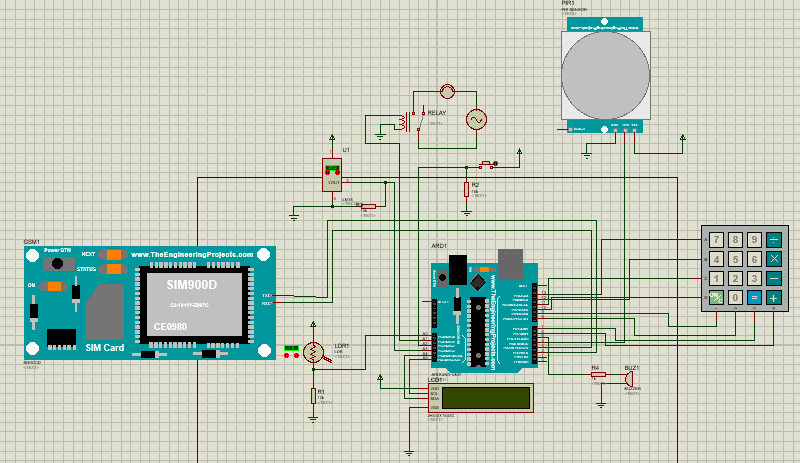
MasterMind Game
Duration: -
A fun code-breaking game implemented in C++, where players guess a secret code of colors based on clues. The game provides hints such as R, W, and - to help the player guess the correct sequence.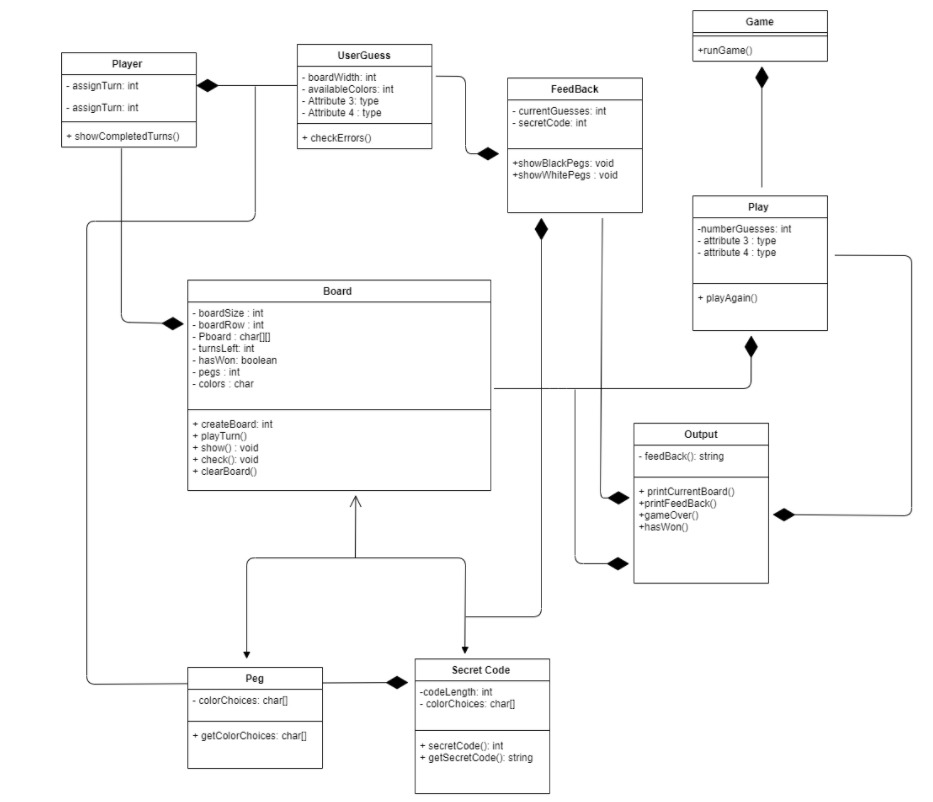
publications
(In Peer Review - ID 1598979 @Frontiers in Medicine) Automatic Extraction of SmPC document for IDMP data model construction using Open-Source Foundation Model LLM RAG: A preliminary experiment for Pharmaceutical Regulatory Affairs.
Published Tentatively July 2025
This research introduces an automated approach using open-source Large Language Models with Retrieval-Augmented Generation (RAG) to extract critical data from SmPC documents for building the IDMP data model, crucial in pharmaceutical regulatory compliance. The study aims to streamline and enhance the accuracy of data extraction for regulatory affairs, reducing the manual workload in aligning with IDMP standards.
Recommended citation: EU ISO IDMP IG Chapter 2: Data elements for the electronic submission of information on medicinal products for human use. European Medicines Agency, v2.1.1.
talks
Bridging the Compliance Gap: IDMP Gap Analysis for the transition from xEVMPD to PMS
Published:
With the ongoing migration from the eXtended EudraVigilance Medicinal Product Dictionary (xEVMPD) to the Product Management Service (PMS), companies face the added challenge of ensuring seamless data integration and synchronization.
